EN
2023-04-12

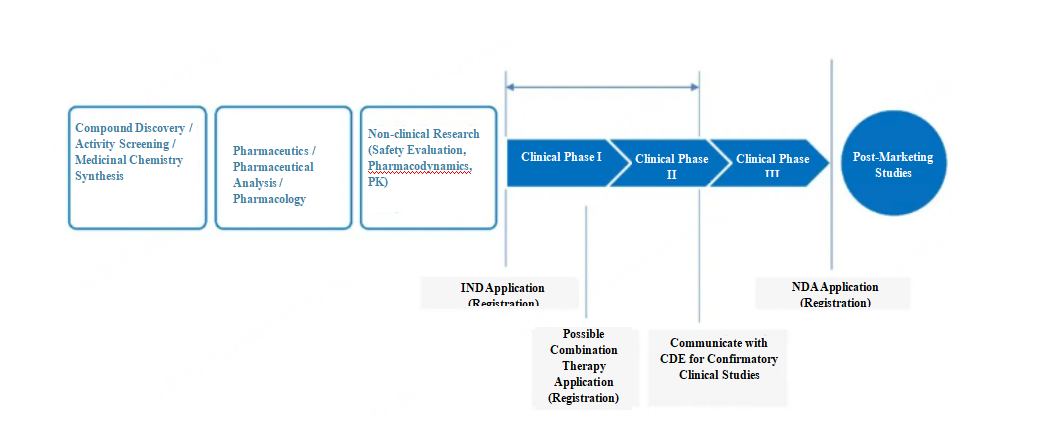
From April 7 to 9, 2023, the China Innovative Drug (Device) Medical Conference and CMAC Annual Meeting were successfully held at the Suzhou International Expo Center. With the theme "Innovation Restarts, Medicine Runs Throughout the Process", the conference brought together over 5,000 industry experts and colleagues from the medical and pharmaceutical fields to exchange trends and challenges in new drug development, regulatory registration, medical affairs, and more, focusing on clinical value orientation, medical value as the foundation, and adherence to correct guiding principles as the core, aiming to concentrate on medicine and research, scientific and technological innovation, and talent innovation with a more forward-looking perception and pragmatic attitude.
UnionClin made its grand debut at this annual meeting. We extended our gratitude to all industry colleagues for coming to the exhibition and for their support and attention to UnionClin! In the exhibition, UnionClin showcased its integrated service from the strategy to implementation for clinical studies, which focuses on oncology, infectious diseases, and autoimmunity and aims to provide leading technology and professional services to help more clinically valuable new drugs reach the market as soon as possible.

New Drug R&D Thematic Conference: Future Development and Considerations of Clinical Research on China's Innovative Drugs

○ Key Decision-Making in Early Clinical Development of Innovative Drugs
Haixiong Han, Chief Medical Officer of UnionClin
Phase I clinical studies play a pivotal role in the entire chain of new drug development, linking non-clinical studies and Phase II clinical studies. In his keynote speech on "Key Decisions in Early Clinical Development of Innovative Drugs," Dr. Haixiong Han pointed out that poor design in Phase I clinical studies may cause the study to terminate early during dose escalation due to safety issues, such as overly stringent DLT (Dose-Limiting Toxicity) design leading to the inability to escalate. Incorrect dose selection in Phase I may fail to observe efficacy in Phase II, causing the study to terminate; for example, setting the RP2D (Recommended Phase II Dose) too low may lead to no observed efficacy. Therefore, Phase I clinical studies are extremely important.
The Role of Phase I Study in the Entire New Drug R&D Process
In the design of Phase I clinical study protocols, the dosing regimen is a very important aspect. In the process of optimizing the dosing regimen, clinical pharmacology can provide significant assistance. Taking the case of tepotinib as an example, tepotinib was marketed for the treatment of MET exon 14 skipping mutation non-small cell lung cancer. In the early dose-escalation phase, within a wide dose range of 30-1400mg QD administration, MTD was not obtained. In the decision-making process of determining the RP2D, modeling, and simulation techniques were mainly used to support dose selection. The actual modeling was conducted in two parts. First, through a translational quantitative PK/PD model from non-clinical studies, the relationship between PK and MET phosphorylation inhibition was clarified: when pMET inhibition reached 95%, tumor growth was completely controlled. This suggested that in clinical development, pMET inhibition reaching 95% could be used as the standard for selecting an efficacious dose. Subsequently, based on clinical data obtained, the human PK/PD relationship was validated, and the pMET inhibition under different dosing regimens was simulated. Finally, 500mg was determined as the RP2D, much lower than the MTD, and subsequent clinical studies also showed that the 500 mg dose was safe and effective.
Optimization of Phase I Dosing Regimen
During Phase I clinical studies, sponsors are very concerned about when to stop dose escalation. From a medical perspective, dose escalation can be terminated once the investigational drug reaches the MTD. Most small-molecule drugs can identify an MTD, but biologics often do not have an MTD and may terminate dose escalation when PK becomes nonlinear or receptor occupancy is saturated. Whether PK nonlinearity occurs or receptor saturation is reached must be determined with the involvement of clinical pharmacology.
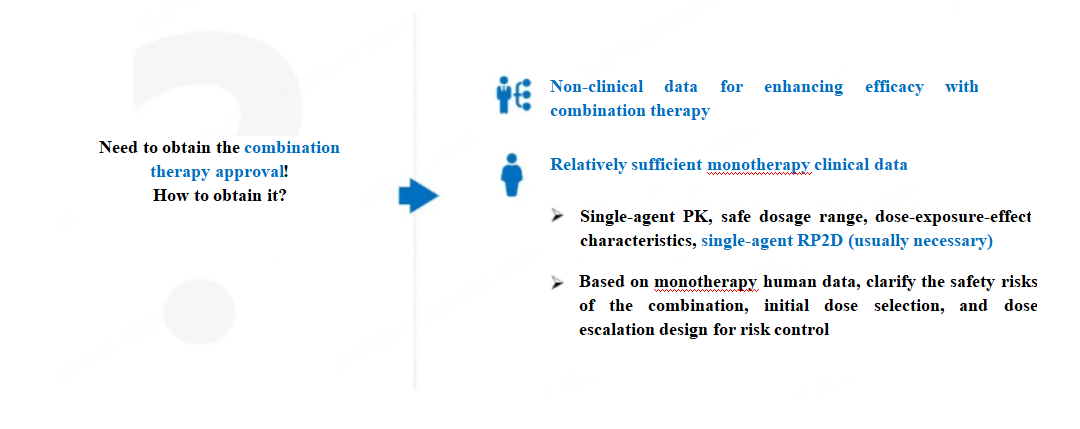
Currently, combination therapies are still frequently used in Phase I clinical studies of antitumor drugs. This requires sponsors to provide non-clinical data on the synergistic effects of the combination and relatively sufficient single-agent clinical data to obtain the combination therapy approval from the CDE. So what constitutes relatively sufficient single-agent clinical data? Generally speaking, it involves obtaining data on single-agent PK, safe dosage range, dose-exposure-response characteristics, and single-agent RP2D, to clarify the safety risks of the combination, starting dose selection, dose escalation design, and risk control. Sponsors must draft and submit the combination therapy protocol to the CDE to obtain the combination therapy approval.
Combination Therapy
As Phase I dose escalation nears completion, an issue that still needs to be addressed is the selection of expansion cohorts, especially for anti-tumor drugs. Determining the indications for expansion cohorts requires comprehensive consideration of many factors, such as indications where competitors have shown efficacy, indications where the investigational drug has better non-clinical pharmacodynamic results, indications with significant ethnic differences where efficacy is average abroad but may be better domestically, indications with low incidence abroad but high incidence domestically and potentially effective mechanistically, and so on.
Based on years of experience in the early clinical development of new drugs, Dr. Haixiong Han considered that early clinical studies must involve rapid trial and error and dynamic decision-making, considering several key factors: First, choosing the right PI is half the success; second, selecting the right patient population is the guarantee for approval; third, optimizing the dosing regimen is a critical step—clinical pharmacology is very important; fourth, biomarkers are becoming increasingly important—basket trials that select patients based on biomarkers may allow the investigational drug to be approved across multiple tumor types; fifth, entering the next clinical phase at the appropriate timing is a good opportunity to overtake; sixth, if the sample size is too large, progress is slow; if the sample size is too small, results may not be accurate.


Shanghai UnionClin Co., Ltd (UnionClin) was founded in 2017. The headquartered is based in Shanghai. UnionClin focuses on oncology, infectious diseases, and autoimmunity, and delivers an integrated service from the strategy to implementation for clinical studies of new drugs. Our services include clinical strategy consultation, protocol design, registration, pharmacovigilance, data management and statistics, clinical operations, independent medical imaging assessment, bioanalysis, clinical pharmacology, on-site management services at study sites, and personnel outsourcing. UnionClin’s team members are nearly 700, distributed across more than 25 cities nationwide.
UnionClin upholds the mission of "accelerating clinical studies for new drugs to bring new life to thousands of patients", continuously building clinical research networks and professional service systems, and has provided services for more than 180 projects. They have established extensive collaborations with clinical research experts and clinical research centers, dedicated to helping clients design and deliver clinical studies, enhancing the accessibility of clinical research and patient participation, and committed to promoting the early market entry of more clinically valuable new drugs.
UnionClin values its commitments to clients by integrating our professional experience in clinical development, Phase I-III clinical research services, integrated digital tool systems, and commercialization services to form end-to-end full-process solutions. We continuously reduce R&D costs, improve R&D efficiency, and help clients and partners realize their R&D dreams.