EN
Providing development and iteration of quality and efficiency improvement tools based on the bottleneck or pain point scenarios throughout the entire process of new drug clinical research
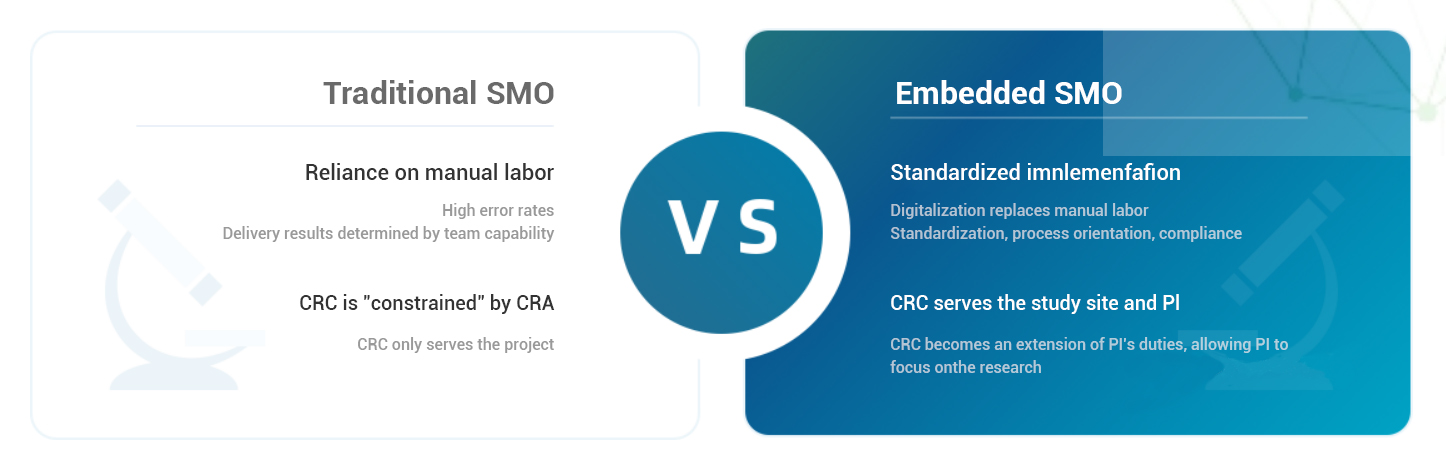
YaoYan Intelligent, supported by systems and technology, driven by the needs of investigators and research centers, and centered on project progress and quality, adopts an innovative "System + CRC" model to carry out deep cooperation with hospitals and experts, creating rapid, efficient, high-quality, and professional digital embedded on-site services for clinical research centers (SMO).
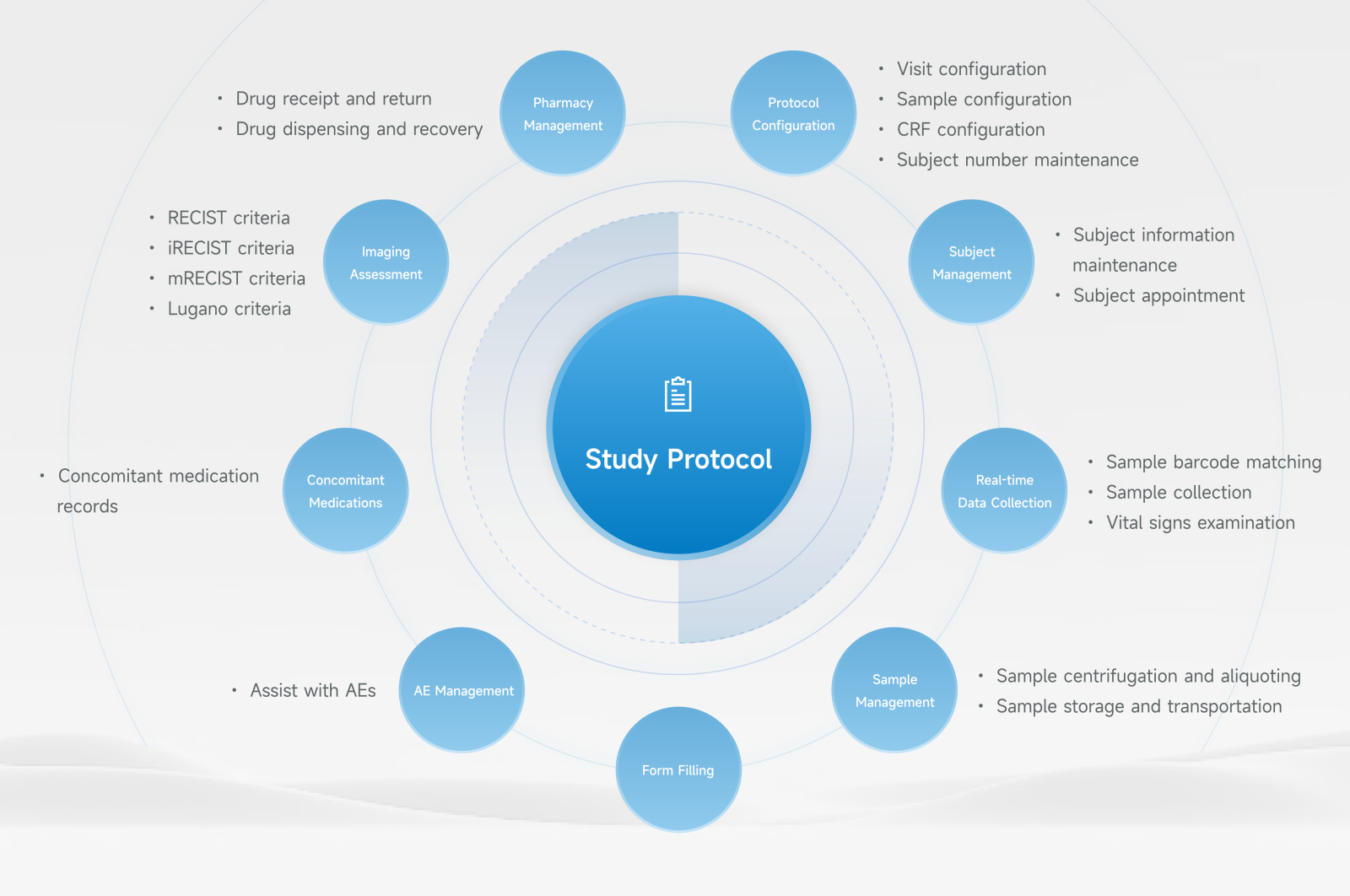
The technical development team of YaoYan Intelligent, together with clinical experts, jointly developed eDISC—the intelligent clinical trial management system for research centers. Through intelligent and informatization technologies, the system replaces a large amount of manual work, helping clinical expert teams to expand management boundaries and collaboratively manage large-scale multicenter clinical trials. It can dynamically update and adjust clinical trial progress in real-time, effectively improving study efficiency. It forms a clinical trial "Internet of Things" with automatic data collection and automated workflow management, achieving unification of GCP, facilities, personnel, data, and operational standards.
Currently, we have provided eDISC system construction services to many hospitals including Shanghai East Hospital, Beijing Cancer Hospital, Blood Diseases Hospital of Chinese Academy of Medical Sciences, Sun Yat-sen University Cancer Center, Chongqing University Cancer Hospital, Shandong Cancer Hospital, The First Affiliated Hospital of Bengbu Medical University, The Second Hospital of Dalian Medical University, among others.
START Shanghai Co., Ltd. is an SMO focusing on early-stage clinical research of oncology, providing research center management and clinical research coordination services based on the "Collaborative Platform for Early-stage Anti-tumor Clinical Research".
By introducing the mature management system from the START USA program for early-stage clinical research of new anti-tumor drugs, and drawing on its practical experience, START aims to build an international clinical research service platform.
All core members have received standard training from START USA. START has established an embedded cooperation model with seven centers, including Shanghai East Hospital, Shandong Cancer Hospital, the First Affiliated Hospital of Nanchang University, and the Harbin Medical University Cancer Hospital, etc. START has cooperated with over 180 clients and has successfully completed 308 research projects, including 210 multicenter leading projects, over 80 first-in-class projects, 41 international multicenter projects, and 11 overseas projects, and 8 products have been launched successfully (data as of December 31, 2024).
A joint venture subsidiary of UnionClin and Hongren Pharmaceutical, providing clinical bioanalysis and pharmacokinetic research services for various chemical drugs and biological products.
Laboratories are established in Shanghai, Wuhan, and Guangzhou, featuring GLP-compliant bioanalysis platforms equipped with over 200 analytical instruments. The subsidiary has undertaken more than 500 clinical biological sample analysis service projects, and developed over 300 drug analysis methods. More than 100 projects have passed on-site CFDI inspections, and over 20 service projects have completed simultaneous submissions in both China and the United States.
 |
 |
 |
 |
 |
ClinElite (Shanghai) Pharmaceutical Research and Development Co., Ltd. offers FSP service of clinical research talent, and provides clients with one-stop talent resource solutions.
In response to the request of pharmaceutical companies at differentstages for clinical research personnel allocation, cost and risk control, ClinElite provides services relating to FSP of clinical research professionals, clinical operation consultation, quality control, and training, to improve the flexibility of human resources for pharmaceutical companies. ClinElite has established cooperation with over 50 clients, with over 200 outsourced positions. Our clinical research personnel has been dispatched to companies such as TopAlliance, Henlius, InnoCare Pharma, Legend Biotech, Everest Medicines, Inventis Bio, Huadong Medicine, AnHeart Therapeutics, Chipscreen Biosciences, among others.
