EN
2025-01-16
Union Laiya
Established in 2019, Union Laiya (Shanghai) Data Technology Co., Ltd., a subsidiary of Shanghai UnionClin Co., Ltd., is dedicated to providing comprehensive professional biometric services to domestic and international innovative drug, vaccine, and medical device companies, encompassing clinical trial statistical consultation, trial design and protocol writing, data management, statistical analysis and programming, and more. Our team comprises dozens of core technicians and experts with an average of around ten years of industry experience, hailing from renowned pharmaceutical companies and clinical CROs both domestically and internationally.
As Union Laiya continues to strive for excellence and deepen its expertise, we are pleased to announce that the company has officially become a gold member of CDISC (Clinical Data Interchange Standards Consortium), demonstrating our relentless pursuit of standardized, efficient, and international clinical research data standards.
Dr. Zibao Zhang, General Manager of Union Laiya, who previously served as the Chairman of CDISC China (C3C) and CDISC Asia Pacific (AP3C), has been actively promoting this global standard to facilitate standardized and efficient full-process clinical trial data. With over 20 years of experience in the biomedical industry, including technical team management, business development, venture capital, and corporate management, Dr. Zhang has been responsible for establishing and managing statistical teams in China for several multinational clinical CRO companies. He now focuses on corporate management, striving to build an international brand and provide high-quality clinical CRO services to clients world wide. Dr. Zhang holds a Ph.D. from Fudan University, a Global EMBA degree from China Europe International Business School(CEIBS). The core members of Union Laiya's statistical analysis and programming team possess years of experience in the pharmaceutical industry, as well as expertise in CDISC standard consultation, training, and implementation, having supported multiple domestic and international pharmaceutical companies in CDISC standard-based electronic data submissions (e-submission data package).
As an authoritative global standards development organization in the field of clinical research data, CDISC provides a comprehensive set of unified data standards and management norms for pharmaceutical industry and research institutions worldwide, as illustrated in the figure below.
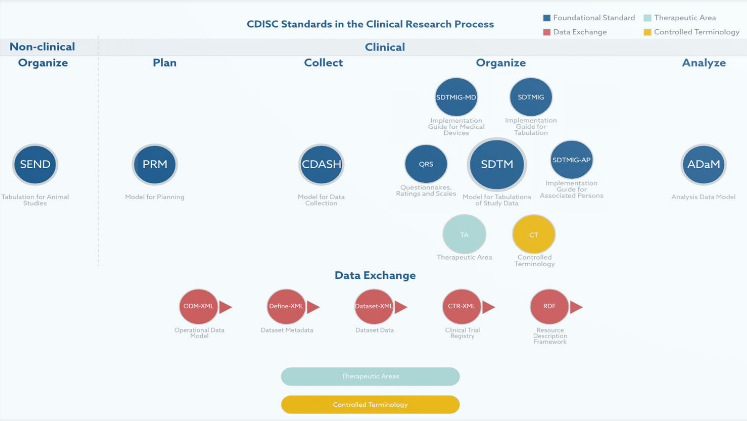
Figure CDISC Standards(https://www.cdisc.org/standards)
Becoming a CDISC corporate member means Union Laiya will bring the following series of benefits to our clients.
Enhance Data Quality and Consistency
By adhering to CDISC standards, enterprises can ensure higher consistency and accuracy in the data collected during clinical trials and other research. This helps reduce data errors, duplicates, and redundancies, thereby improving data quality.
Simplify Data Exchange and Integration
CDISC standards facilitate data exchange and integration between different systems. This enables enterprises to more easily migrate data from one system to another or integrate data from various sources for analysis.
Accelerate Drug Development Process
By using CDISC standards, enterprises can streamline the collection, management, and analysis of clinical trial data, thereby accelerating the drug development cycle. This helps enterprises launch new drugs and treatments more quickly to meet market demands.
Reduce Compliance Risk
Adhering to CDISC standards helps enterprises meet regulatory compliance requirements and reduce compliance risks arising from data issues. This is particularly important for enterprises conducting business globally.
Facilitate Data Sharing and Collaboration
CDISC standards promote data sharing and collaboration among different organizations. This allows enterprises to more easily share data with partners, regulatory agencies, and other stakeholders, jointly advancing scientific research and medical progress.
In the days to come, we will continue to uphold the principle of "Client-Centric, Data-Driven" continuously enhancing the management level and service quality of clinical research data to provide our clients with the highest quality services and most thoughtful solutions. Choosing UnionClin Laiya means choosing a guarantee of quality and innovation. We look forward to working with you hand in hand to build a new future for clinical research data.
Written by: Union Laiya Statistical Analysis and Programming Team